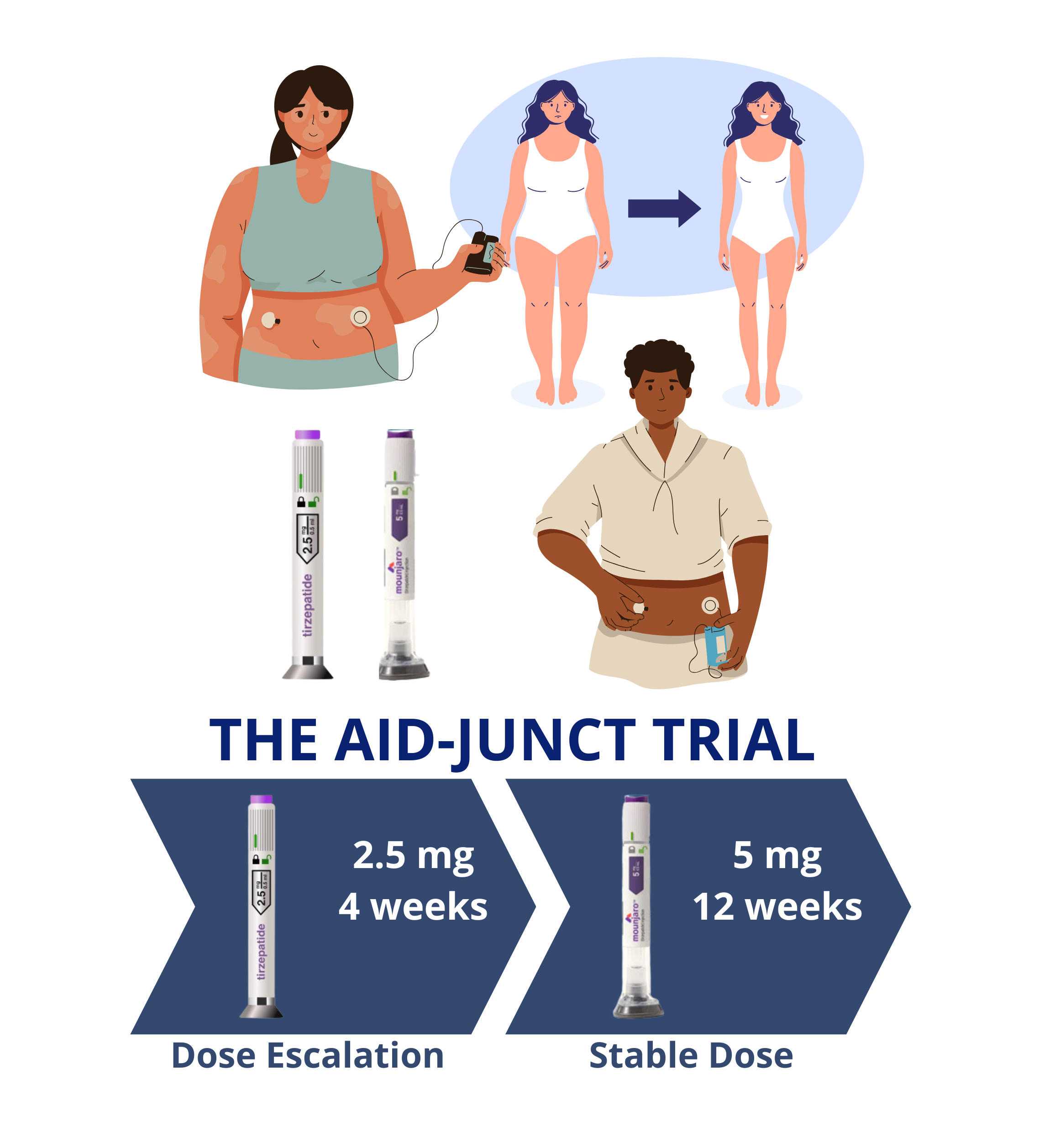
AID-JUNCT study: GIP/GLP-1RA as Adjunctive to Automated Insulin Delivery
Findings from a prospective, randomized clinical study
Innovative approaches to combination therapy in type 1 Diabetes
Glycemic control in type 1 diabetes (T1D) remains a challenge, with ~32% of adults in Switzerland achieving an A1c target of <7%. The dual glucagon-like peptide-1 receptor agonists (GLP-1RAs) and glucose-dependent insulinotropic polypeptide (GIP)/GLP-1RAs has emerged as promising therapy in T1D. Retrospective studies have shown people with T1D can significantly improve glycemic control with a reduction in insulin dose and body weight when long-acting GLP-1RAs or GIP/GLP-1RAs are added to insulin therapy.
This study will assess glycemic control in people with T1D on a stable dose of long-acting GIP/GLP-1RA (Tirzepatide) as an adjuvant therapy to automated insulin delivery (AID). We are conducting a prospective, parallel, open-label, randomized clinical trial to test the hypothesis that available AID systems combined with GIP/GLP-1RA will lead to safe and more efficacious glycemic control than Standard of Care (SoC) during the assessment period. The outcome of this study will provide unique data about the safety and efficacy of using GIP/GLP1-RA as adjuvant therapy to AID systems.
Sponsor: Prof. José García-Tirado, PhD – University of Berne
Principal investigator: PD Dr. med. Thomas Züger, Chief Physician – Kantonsspital Olten
PhD student: Carolina Fragozo-Ramos
Funding: Internal funds
More recent projects
Insulin Activity Project
Exploring Insulin Stability and Activity in Diabetes ManagementInsulin is essential for diabetes management, but its...
Obesogenic microbiota Project
Exploring the connection: obesity, gut microbiome, and metabolic healthCause and effect relationship between obesity...
Bacterial metabolites modulating Immune Function
Exploring microbial metabolites and immune activationUnveiling the role of bacterial metabolites in dendritic cell...


DCB Research AG
Freiburgstrasse 3
3010 Bern
Switzerland